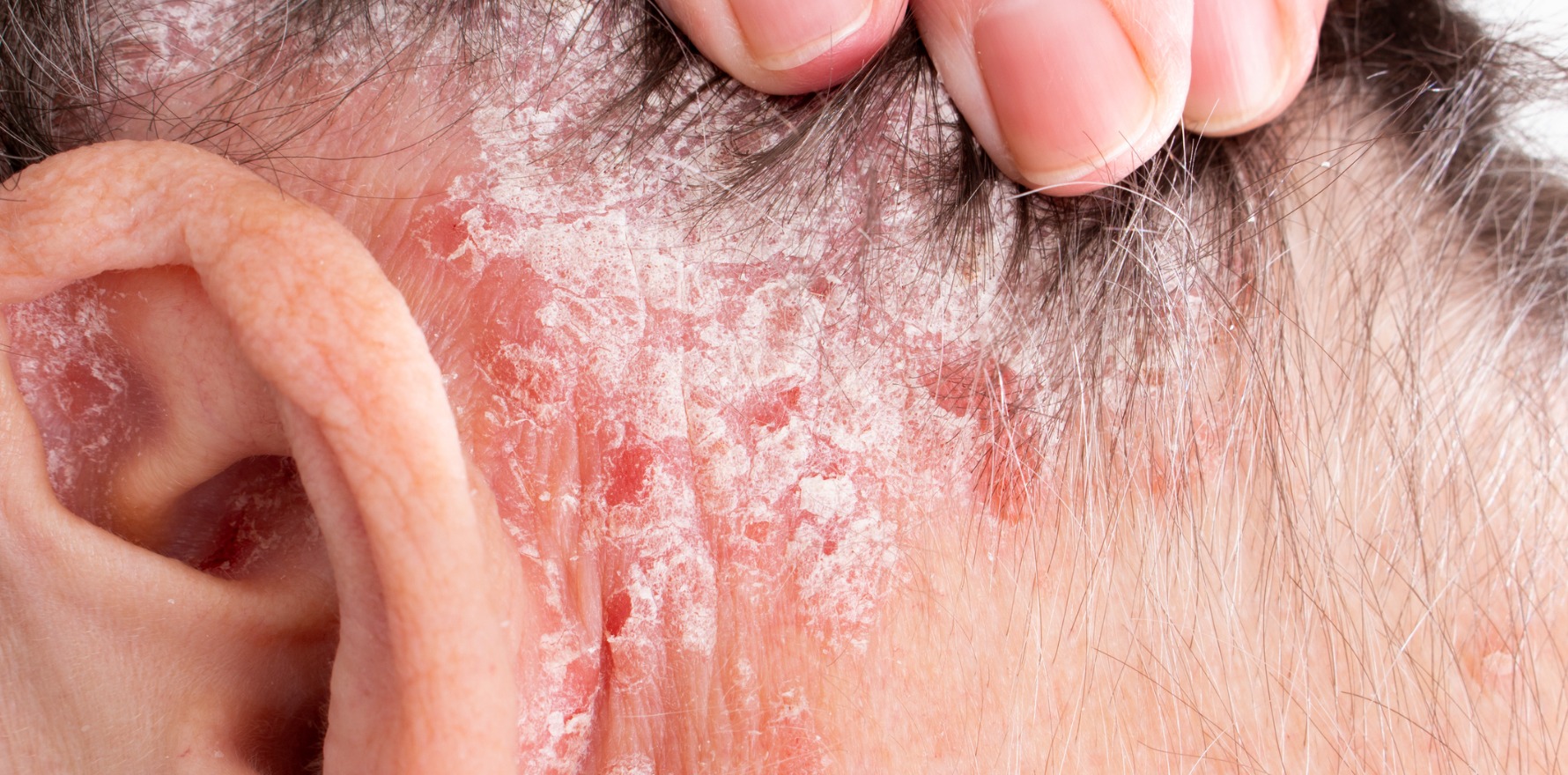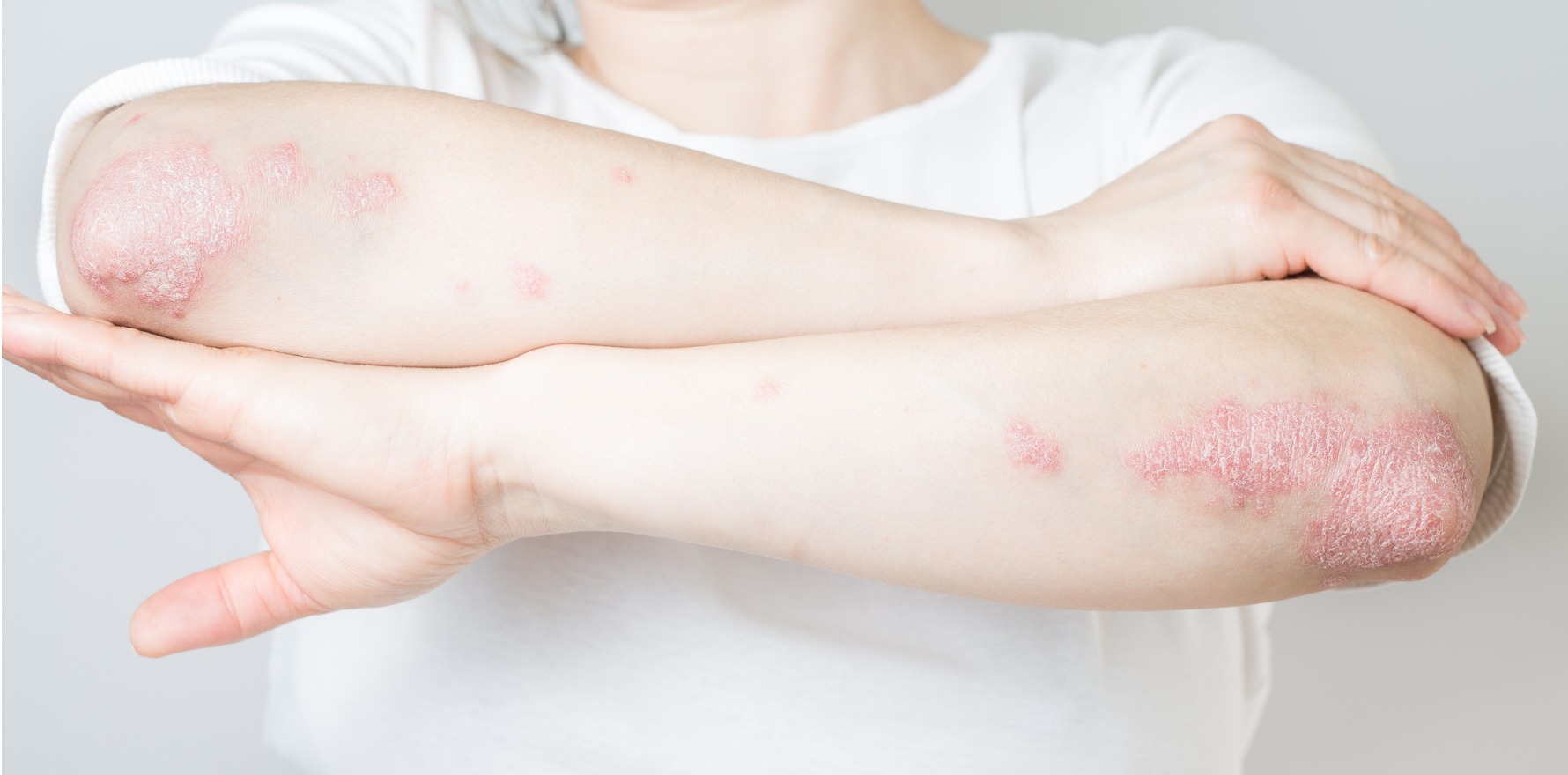
Deucravacitinib can have a large benefit for patients with less severe disease, a new study finds.
New research suggests less severe psoriasis responds well to deucravacitinib treatment in the short term.
Previous research into the effects of deucravacitinib on psoriasis – namely the POETYK PSO-1 and PSO-2 phase three clinical trials – had focused on patients with at least 10% of their body surface area affected by the condition.
A poster presented at last month’s Maui Derm Hawaii conference suggests the treatment suggests the oral tyrosine kinase 2 inhibitor can reduce the condition’s impact on quality of life in patients with less severe psoriasis.
“This study highlights the negative impact of psoriasis on our patients’ quality of life – but more importantly we learned that even patients with low body surface area involvement are affected by their disease negatively,” Professor Leon Kircik, a dermatologist at Mount Sinai in the US who was involved in overseeing the new research, told Healio.
As part of the phase four ARTISTYK study, 180 patients with as little as 3% of their body surface area affected by psoriasis received deucravacitinib or placebo in a 2:1 fashion.
Patients who received deucravacitinib were three times more likely to achieve better scores on the Dermatology Life Quality Index after 16 weeks of treatment than patients who received the placebo.
One in three patients in the deucravacitinib group reported they experienced zero effects of psoriasis on their quality of life after 16 weeks of treatment.
Furthermore, over half of the deucravacitinib patients who indicated that their psoriasis had a “very large or extremely large” impact on their quality of life before starting the trial (i.e., a DLQI score of 11 or more prior to treatment) reported a “significant decrease” after 16 weeks, compared to 18% of patients who received placebo.
Professor Kircik said the results served as an important reminder that disease severity does not always reflect the impact on the patient.
“I think it is important to remember that the impact on QoL is not dependent upon the disease severity and even mild disease may have negative effects on our patients and therefore appropriate treatment is important,” he said.
The TGA approved deucravacitinib as a treatment for moderate to severe plaque psoriasis in adult patients who are candidates for systemic therapy or phototherapy in 2022, before it was added to the PBS in October 2023.
The PBS updated the streamlined authority listing last year, allowing rheumatologists and GPs to prescribe subsidised deucravacitinib provided the patient meets certain criteria.
“The main benefit of this broadening of streamlined prescribing is that rheumatologists are able to prescribe these agents in patients with moderate-to-severe skin psoriasis, in order to optimise their skin and joint disease activity,” Dr Anna Antony, a consultant rheumatologist at Monash Health, told Dermatology Republic after the PBS changes.
“This is particularly beneficial for patients who do not meet PBS criteria for a bDMARD/other tsDMARD on the psoriasis or psoriatic arthritis pathways or have difficulties accessing a dermatologist.”