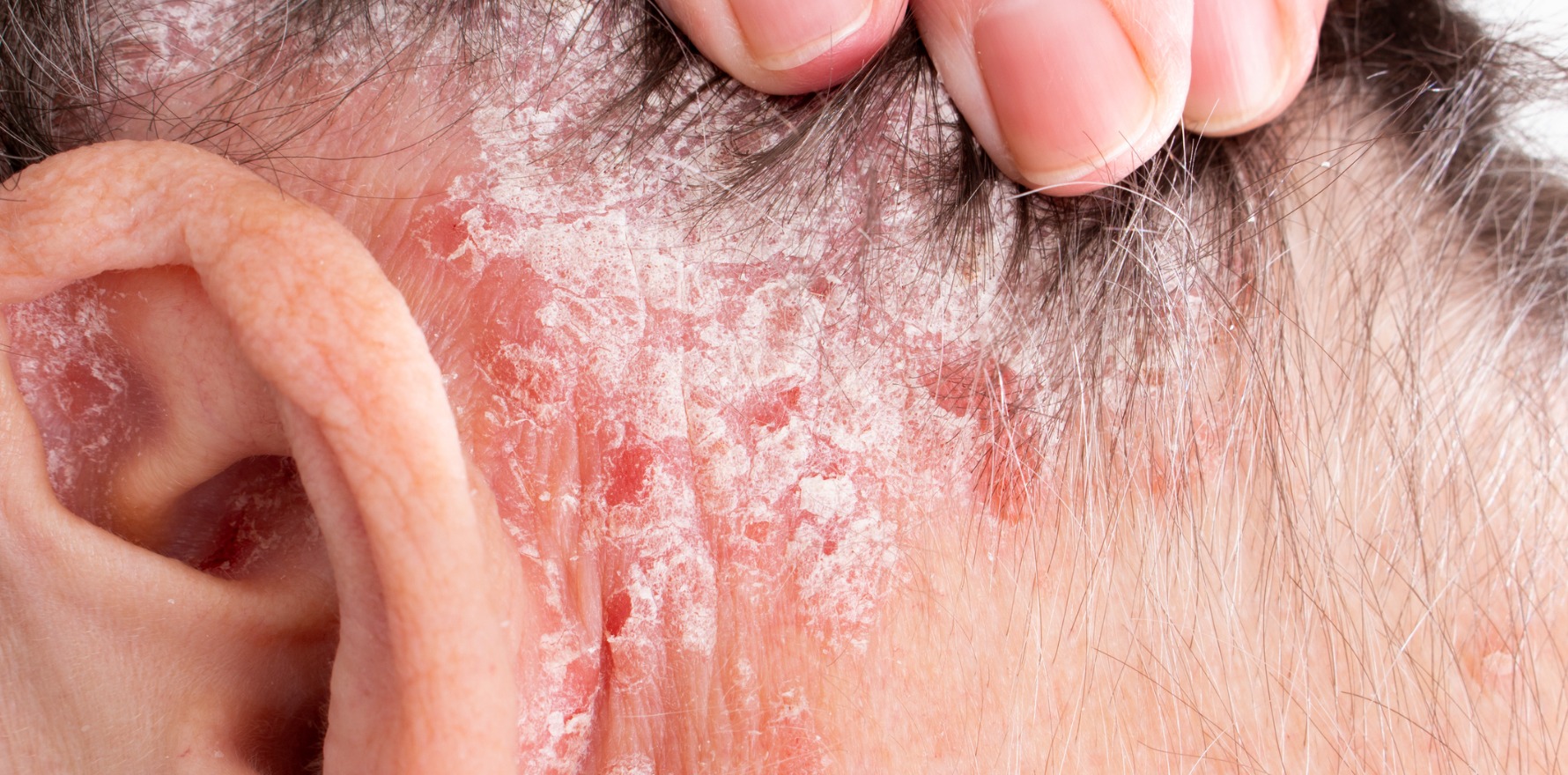
Patients receiving immune checkpoint inhibitors were three times more likely to develop psoriasis compared to patients receiving traditional chemotherapy.
New research suggests ICIs lead to psoriasis, potentially as a result of T cell activation.
Immune checkpoint inhibitors have been a game-changing cancer therapy, but their use is associated with significant concerns about immune-related adverse events across multiple organ systems, including the lungs, gastrointestinal tract and skin. The pathological mechanism underlying ICI-related adverse events is thought to be similar to that of psoriasis, yet it is unclear if and how ICIs impact the risk of developing psoriasis.
But a recent Taiwanese study, published in JAMA Dermatology, explored the association in a large cohort of cancer patients and found that ICIs doubled or tripled the likelihood of psoriasis.
“Although this adverse effect is relatively uncommon, it is important for medical professionals, clinicians and caregivers to be aware of this potential risk to improve skin health and ensure optimal cancer care,” the researchers write.
Data on over 135,000 patients with stage III or IV cancer who were treated with an antineoplastic agent over a 2.5-year period were obtained from the National Health Insurance database and cancer registry in Taiwan. Patients were followed for up to three years. Individuals with a prior history of psoriasis were excluded from the study.
The majority of patients were treated with chemotherapy or another target therapy (132,042; 97.6%) rather than ICIs (3188; 2.4%). Three quarters of patients prescribed ICIs received them as monotherapy. The remaining patients received a combination of ICIs and targeted or cytotoxic therapies.
During the follow-up period 295 patients (0.2%) were diagnosed with psoriasis; 16 ICI users (0.5%) and 279 non-ICI users (0.2%). After adjusting for demographic characteristics, comorbidities and outpatient visits, ICI users were 3.5 times more likely to develop psoriasis compared to non-ICI users.
The risk of developing psoriasis was highest in the first 180 days of starting to use ICIs. Additional subgroup analyses revealed that ICI users over the age of 65 were 7.9 times more likely to develop psoriasis compared to non-ICI users, and that male ICI users were 3.6 times more likely to develop psoriasis compared to non-users.
While the researchers were not able to confirm what was driving this association, they weren’t completely in the dark on the matter.
“ICIs block inhibitory pathways, such as PD-1/PD-L1 and CTLA-4, which cancer cells use to evade the immune system, thereby leading to the reactivation of T cells. This may enhance immune activity and can quickly lead to inflammation and autoimmunity, manifesting as psoriasis,” they concluded.
However, the researchers were also quick to point out that their findings needed to be interpreted with caution, as they did not have access to key confounders such as lifestyle and genetic factors, BMI and environmental exposures – all things that could affect the incidence of psoriasis.
“Oncologists should monitor for early signs of psoriasis after starting ICIs, educate patients to report skin changes promptly and collaborate with dermatologists on how to best manage psoriasis without disrupting cancer treatment,” Assistant Professor Shoshana Marmon, a US-based dermatologist who was not involved in the current study, told MedPage Today.




